— This article by Jerry Cates and Marvin W., first published in November 2008, was last revised on 24 April 2016. © Bugsinthenews Vol. 09:11(01).
—————————————–
The photo on the right was taken by Marvin W., in Kempner, TX on 11.12.08. I have taken the liberty of applying image enhancement software to it and the other photos provided on this post to lighten the coloration and bring out some of the subtle features that would otherwise be lost. If you find a trapdoor spider like this in the field, it will be much darker, to the point of appearing almost entirely black.
Notice that the photos on this page, as with all the photos posted on bugsinthenews.info, can be enlarged for more detailed viewing by placing your cursor over the photo and left-clicking.
Marvin and his wife recently moved to Texas from the frigid northwest (Washington state). Not being familiar with the size of our Texas tarantulas, he first suspected this to be one. As both are orthognaths in the infraorder Mygalomorphae, they share a number of gross anatomical features. However, the leg span of this male (note the swollen distal palps) is about half that of the average Texas tarantula.
In the following narrative, replete with photos, we will refer to anatomical characters that bear on its identity, as described by a number of authorities, including the key provided in Ubick 2005, p. 25-37. Major changes to the taxonomy of the Mygalomorph spiders have recently taken place that must be taken into account before we can arrive at a conclusion regarding this spider’s taxonomical identity. In fact, because we do not have definitive images of this specimen’s genitalia, and no effort was made to find and examine its burrows, arriving at a firm conclusion in this regard is practically impossible. Regardless, much can still be said about this spider.
Marvin’s wife, a now-repentant arachnophobe, sprayed the first of these spiders–which she found on their back patio just after a cool, autumn rain–with a pesticide. The next morning Marvin found this one, on the same patio. He captured it and took several photos.
After looking Marvin’s photos over, I asked to borrow the spider for microscopic work, but this one had already been released. Marvin kindly retrieved the remains of the first for me to work with, and the micrographs that follow were taken from that specimen.
This photo, taken of the first spider Marvin’s wife found on their patio, shows the spider’s head and thorax, i.e., the cephalothorax, or dorsal prosoma, covered by a sclerotized plate, the carapace.
Also is shown the forward part of the dorsal abdomen, and the proximal three segments of the legs and pedipalps.
Notice the robust jaws (chelicerae) that project outward as a forward extension of the face.
This structure typifies spiders in the infraorder Mygalomorphae.
As noted in Ubick, 2005, pg. 25, such spiders also have two pairs of book lungs and eight eyes, are without the anterior median spinnerets (AMS) found in many araneomorphs, and have stout legs.
The cephalothorax is smooth and glabrous. Notice the deep, procurved thoracic furrow (an important, though not definitive, anatomical marker for the Ctenizidae) that separates the slightly bulging head (pars cephalica) from the thoracic region (pars thoracica) lateral and posterior to it.
The musculature for the spider’s sucking stomach attaches at the thoracic furrow, to the underside of the carapace.
A portion of the lateral anterior abdomen is a lighter color, due in part to a paucity of hairs over the spider’s anterior book lungs. The abdomen has no dorsal tergites, which rules out the Antrodiaetidae, Atypidae, and Mecicobothriidae.
The photo at left shows the spider’s ventral body. Notice that the chelicerae and fangs are paraxial, opening more like claws than scissors (as in the araneomorphs).
Most mygalomorphs burrow in the ground. Their claw-like fangs assist in excavating their burrows.
The palpal endites of this specimen are similar in structure to the coxae of the legs, longer than wide, and are lined medially with a loose row of bristles known as scopulae (not to be confused with the dense pads on the ventroapical surface of the legs of some spiders, which are also referred to as scopulae) that surrounds the mouth.
The entire anterior portion of the ventral abdomen is occupied by a pair of book lungs, whose lateral extension is visible on the dorsal abdomen in the photo just above this one.
A second pair of book lungs is positioned on each side of the ventral abdomen, posterior to the first pair, as indicated by the light-colored regions there.
Notice the spinnerets on the posterior abdomen.
Most mygalomorphs, including this specimen, have two pairs of functional spinnerets (the PMS and PLS), while araneomorphs have a third pair (the ALS) that is absent (lost) in the mygalomorphs.
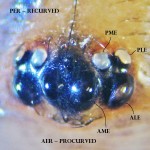
The eye cluster of this spider is shown in the midsection of this photo, with the basal chelicerae, in the lower portion of the photo, out of focus.
The eyes are arranged in two rows, an anterior (foremost) row nearest the chelicerae, and a posterior (rearward) row behind it.
Each row is, by convention, divided into median (midmost) and lateral (outermost) eyes, and are referred to in coded form.
Notice the two anterior median eyes (AME) in the center of the face, facing forward and slightly upward. Flanking the AME, the anterior lateral eyes (ALE) face forward, angled to the side.
Notice that, in this spider, the posterior eyes form a triangle with the ALE, as the anterior eyes form one with the PME.
The posterior median eyes (PME) are brightly reflective. Notice also that the PME face upward, slightly back and toward a point above the centerline of the body. The posterior lateral eyes (PLE) face backward and to the side.
This eye arrangement does not provide acute vision, as found in jumping or wolf spiders, but does afford a wide field of view.
The lenses are fixed, but the retinas are moveable, so the spider can examine a large expanse of its environment without moving its head.
The eye cluster is now shown from the front.
Note the basal chelicerae in the lower portion of the photo.
Separating the eyes and chelicerae is a delicately delineated clypeus (the membrane connecting the basal chelicerae to the carapace).
Note that the head (pars cephalica) is not elevated behind the eye group, as in the Ummidia (Ctenizidae).
The anterior median eyes (AME) in the center of the face look forward and slightly upward.
Flanking the AME, the anterior lateral eyes (ALE) face forward, angled down and to the side.
The brightly reflective posterior median eyes (PME) are midway between, and behind, the ALE and AME.
The posterior lateral eyes (PLE), which face backward and to the side, are not visible but their tubercles appear as dark knobs, next to the PME.
A close-up view of the fangs, endites, and the anterior labium (the lip) of this spider is shown here.
Endites (the two leg-like structures forming a “V” at center left of the photo, which are actually the proximal segments of this spider’s pedipalps) are generally an expanded lobe of the palpal coxa, often referred to as gnathocoxa because, in most spiders, they play an active role in food mastication.
In the mygalomorphs, the endites are relatively long and simple.
They hold a morsel of prey in place while it is being masticated by the chelicerae and fangs before being sucked into the mouth.
The endites of this specimen have a thick brush of loose bristles (scopulae) along their medial margins.
The fangs are shown folded into their cheliceral furrows, and are flanked laterally by a row of denticles (diminutive teeth), and medially by a row of more substantial teeth that are barely visible in this perspective. The spider’s mouth is positioned where the distal fangs come together, at the labium, or lip, at the anterior median extension of the sternum (lower mid photo).
As food is masticated by the chelicerae, it is drawn into the mouth by the action of the spider’s sucking stomach.
This photo shows a close-up view of this spider’s distal chelicerae, with the fangs extended (and out of focus) so that the cheliceral furrows and their related denticles, and a rastellum (“little rake,” a rake-like structure near the cheliceral fang base, present in some mygalomorphae) can be examined.
Ubick 2005, p. 270, shows the rastellum as a patch of short spines, which appear to be present in this specimen in the form of hardened tubercles at the medial distal end of each jaw.
So, at least by that latter definition this specimen is positive for that anatomical feature.
This final photo shows a close-up view of the distal fangs, including the orifice through which venom is ejected (injected, actually, once the fangs have penetrated the spider’s prey). In many mygalomorphs, particularly the larger tarantulas, the venom glands are relatively small and reside entirely within the chelicerae.
It would be necessary to dissect this specimen to determine the extent of its venom glands. Many other anatomical features are easily depicted via imagery of the external body, however. These include, for this specimen, details of the distal palps.
—————————————–
Taxonomy:
- Kingdom Animalia (an-uh-MAYHL-yuh) — first described in 1758 by the Swedish taxonomist Carolus Linnaeus (1707 – 1778), using the Latin word animal = “a living being,” from the Latin word anima = “vital breath”, to refer to multicellular, eukaryotic organisms whose body plans become fixed during development, some of which undergo additional processes of metamorphosis later in their lives; most of which are motile, and thus exhibit spontaneous and independent movements; and all of whom are heterotrophs that feed by ingesting other organisms or their products;
- Phylum Arthropoda (ahr-THROPP-uh-duh) — first described in 1829 by the French zoologist Pierre André Latreille (1762 – 1833), using the two Greek roots αρθρον (AR-thron) = jointed + ποδ (pawd) = foot, in an obvious reference to animals with jointed feet, but in the more narrow context of the invertebrates, which have segmented bodies as well as jointed appendages;
- Subphylum Chelicerata (Kuh-liss-uh-RAH-tah) — first described in 1901 by the German zoologist Richard Heymons (1867 – 1943) using the Greek noun χηλη (KEY-lay) = a claw, talon, or hoof + the Greek noun κερας (SAIR-as) = an animal’s horn + the Latin suffix ata — which by convention is suffixed to the names of animal subdivisions — to refer to animals that have specialized appendages before the mouth that they use in feeding, capturing and securing prey and that — in the case of spiders — are further equipped to inject venom and digestive agents into their prey;
- Class Arachnida (uh-RAKH-nuh-duh) — first described in 1812 by the French naturalist and zoologist Jean Léopold Nicolas Frédéric Cuvier (1769 – 1832), usually referred to as Georges Cuvier, using the Greek noun αραχης (uh-RAH-kes) = a spider, in reference to all eight-legged arthropods, including such disparate animals as ticks, mites, scorpions, harvestmen, solpugids, and spiders;
- Order Araneae (uh-RAY-neh-ee) — first described in 1757 by the Swedish entomologist and arachnologist Carl Alexander Clerck (1709 – 1765), who used the Latin word aranea = a spider or a spider’s web, to refer to eight legged arthropods that spin webs;
- Suborder Opisthothelae (oh-PIS-thoh-THEE-lee) — first described in 1990 by the American arachnologists Richard C. Brusca and Gary J. Brusca, who used the Greek words οπισθεν (oh-PIS-thehn) = behind, at the back, yet to come + θηλη (THEE-lee) = nipple or teat, to distinguish this grouping of spiders from the more primitive spiders in the suborder Mesothelae, in that certain characters (e.g., tergite plates, ganglia in the abdomen, and — in particular, inasmuch as the suborder name is a direct reference thereto — median-positioned spinnerets) of the latter are absent in the former; thus spiders in this suborder have spinnerets positioned at the hindmost portion of the abdomen;
- Infraorder Mygalomorphe (my-GAL-oh-MOHR-fee) — spiders with paraxial chelicerae and two pairs of book lungs, as in the more primitive Mesothelae, but without the latter’s tergite plates and most of the latter’s abdominal ganglia, and having their spinnerets positioned at the abdomen’s hindmost portion rather than mid-ventrally as in the Mesothelae; presently comprised of fifteen families:
- Atypidae (Thorell 1870) — 3 genera, 49 species (Platnick WSCv13.5); commonly known as purseweb spiders; 8-27 mm, yellow-brown to dark purple-black in color; the legs of male specimens of Sphodros rufipes (Latrielle 1829) and S. fitchi (Gertsch & Platnick 1980) are bright orange-red;
- Antrodiaetidae (Gertsch 1940) — 2 genera, 33 species (Platnick WSCv13.5); commonly known as foldingdoor, collardoor, or turret spiders (Antrodiaetus), and trapdoor spiders (Aliatypus); 6-26 mm, tan to chestnut brown, with one or more tergites on the anterodorsal abdomen; live in burrows with a flexible collar, a rigid turret, or a trapdoor at the mouth;
- Mecicobothriidae (Holmberg 1882) — 4 genera, 9 species (Platnick WSCv13.5); no common name; mygalomorphs with two tergites on their anterodorsal abdomen (these sclerotized patches may be fused); build sheet webs with silk tubes from sheet to ground that lead into hiding places under terrestrial objects;
- Hexathelidae (Simon 1892) — 12 genera, 112 species (Platnick WSCv13.5);
- Dipluridae (Simon 1889) — 24 genera, 179 species (Platnick WSCv13.5); commonly known as mygalomorph funnelweb spiders; 3.5-17 mm, pale tan to purple-brown in color; thoracic furrow in the form of a short longitudinal groove or a shallow pit or rounded depression;
- Cyrtaucheniidae (Simon 1889) — 10 genera, 102 species (Platnick WSCv13.5);
- Ctenizidae (Thorell 1887) — 9 genera, 128 species (Platnick WSCv13.5); no common name; 10-30 mm or more in length, tan, dark chestnut brown, and black in color; the females lack scopulae, but are equipped with a number of robust lateral digging spines on their pedipalps, as well as on the tarsus, metatarsus, and tibia of legs I and II; carapace generally glabrous, with few distinct spines; thoracic furrow is transverse, typically very deep and procurved; burrows are covered with a thick cork-type trapdoor for all genera, except Cyclosmia Ausserer 1871, which have wafer-type trapdoors;
- Euctenizidae (Raven 1985) — 7 genera, 33 species (Platnick WSCv13.5);
- Idiopidae (Simon 1889) — 22 genera, 314 species (Platnick WSCv13.5);
- Actinopodidae (Simon 1892) — 3 genera, 40 species (Platnick WSCv13.5);
- Migidae (Simon 1889) — 10 genera, 91 species (Platnick WSCv13.5);
- Nemesiidae (Simon 1889) — 43 genera, 364 species (Platnick WSCv13.5); 16-30 mm, golden brown to dark gray, generally concolorous but sometimes with an indistinct chevron pattern on the dorsal abdomen;
- Microstigmatidae (Roewer 1942) — 7 genera, 16 species (Platnick WSCv13.5);
- Barychelidae (Simon 1889) — 44 genera, 307 species (Platnick WSCv13.5);
- Theraphosidae (Thorell 1869) — 124 genera, 946 species (Platnick WSCv13.5);
- Paratropididae (Simon 1889) — 4 genera, 8 species (Platnick WSCv13.5);
- Family not presently determined;
- Genus not presently determined;
- Species not presently determined;
—————————————–
References:
- Beccaloni, Jan. 2009. Arachnids
. Univ. Calif. Press.
- Bond, Jason E. 1994. Seta-Spigot Homology and Silk Production in First Instar Antrodiaetus unicolor Spiderlings (Araneae: Antrodiaetidae). J. Arachnol., 22:19-22.
- Bond, J. E., and Platnick, N. I. (2007). A Taxonomic Review of the Trapdoor Spider Genus Myrmekiaphila (Araneae, Mygalomorphae, Cyrtaucheniidae). American Museum Novitates. Number 3596
- Coyle, Frederick A. 1983 . Aerial dispersal by mygalomorph spiderlings (Araneae, Mygalomorphae) . J. Arachnol., 11 :283-286.
- Coyle, Frederick A. and Wendell R. Icenogle. 1994. Natural History of the Californian Trapdoor Spider Genus Aliatypus (Araneae, Antrodiaetidae). J. Arachnol., 22:225-255.
- Coyle, Frederick A. 2005a. Antrodieaetidae. Ubick, et al., Spiders of North America, an Identification Manual, p. 39-40.
- Coyle, Frederick A. 2005b. Atypidae. Ubick, et al., Spiders of North America, an Identification Manual, p. 41-42.
- Comstock, John Henry. 1912. The spider book: a manual for the study of the spiders and their near relatives
. University of Michigan.
- Emerton, James H. 1902. The Common Spiders of the United States
. Kindle, hardcopy, and paperback editions.
- Foelix, Ranier F. 2011. Biology of Spiders
, Third Ed. Oxford Univ. Press.
- Gertsch, Willis J., 1979. American spiders
. Von Nostrand Reinhold Company.
- Hendrixson, Brent E., and Jason E. Bond. 2005a. Testing species boundaries in the Antrodiaetus unicolor complex (Araneae: Mygalomorphae: Antrodiaetidae): “Paraphyly” and cryptic diversity. Molecular Phylogenetics and Evolution 36: 405–416.
- Hendrixson, Brent E., and Jason E. Bond. 2005b. Two sympatric species of Antrodiaetus from southwestern North Carolina (Araneae, Mygalomorphae, Antrodiaetidae). Zootaxa, 872: 1–19
- Herberstein, Marie Elisabeth (Ed.). 2011. Spider Behaviour: Flexibility and Versatility
. Cambridge University Press.
- Howell, W. M., and R. L. Jenkins. 2004. Spiders of the Eastern United States: A Photographic Guide
. Pearson Edu.
- Jackman, John A. 1999. A Field Guide to Spiders & Scorpions of Texas (Gulf Publishing Field Guide Series)
. Gulf Press.
- Kaston, B. J. 1978. How to know the spiders (The Pictured key nature series)
. WCB McGraw Hill.
- Levi, Herbert W., and Lorna Levi. 1987. Spiders and Their Kin (Golden Guide)
. Golden Press, New York.
- Paquin, Pierre, and Nadine Dupérré. 2003. Guide d’identification des Araignées (Araneae) du Québec. Association des entomologistes amateurs du Québec, p. 50.
- Platnick, Norman I. 2011a. The World Spider Catalog, V. 13.5; CURRENTLY VALID SPIDER GENERA AND SPECIES (Dec. 13, 2012). American Museum of Natural History.
- Preston-Mafham, Rod. 1996. The Book of Spiders and Scorpions
. Barnes & Noble.
- Ubick, Darrell, and Pierre Paquin, Paula E. Cushing, V. Roth (Editors). 2005, Spiders of North America: An Identification Manual
. American Arachnological Society.
- Vincent, Leonard S. 1993. The Natural History of the California Turret Spider Atypoides riversi (Araneae, Antrodiaetidae): Demographics, Growth Rates, Survivorship, and Longevity. J. Arachnol., 21:29-39.
- Wagner, James D., et al. 2003. Spatial Stratification in Litter Depth by Forest-Floor Spiders. J. Arachnol., 31:28-39.
—————————————–
—Questions? Corrections? Comments? BUG ME RIGHT NOW! Feel free to e-mail jerry.cates@entomobiotics.com. You may also leave a comment in the space provided below.
Wafer trapdoor spider (poss. new species), Cresson, TX–24 Feb 2011— BugsInTheNews is a VIEWER-PARTICIPANT WEBSITE. Click on the link for information on what that means.
Dave wrote: I found two of these around my pool skimmer basket yesterday. They are about the size of a quarter. I’m hoping they are southern house spiders and not brown recluses. I don’t see the violin but am having trouble counting the eyes. Thanks- Dave Peters These spiders are light brown, and have a darkened portion on the anterior head slightly reminiscent of the violin marking on the brown recluse, so it is easy to understand why Dave was concerned. Once he had the spiders in custody, and could examine them closely, the vague evidence of a violin marking evaporated. On the other hand,they still looked a bit like the southern house spider. Dave naturally wondered if that might be what they were. I wrote Dave back, explaining that this is a male trapdoor spider in the Cyrtaucheniidae family. The sex was established by the presence of enlarged terminal structures on the pedipalps (the diminutive leg-like appendages stretching out from each side of the spider’s face). Male spiders of most species have enlarged palpal tibias, with a cymbium, a bulb, and an embolus, which together provide for sperm storage and intromission (see the montage of photos later in this article for images of the pedipalps), that transform them into copulatory devices, as described by Foelix (1996), p. 16; the female’s palps, by comparison, are morphologically similar to ordinary ambulatory appendages, except that they are absent a metatarsus. Thus, if a spider has remarkably swollen pedipalps, regardless of the species, it can safely be sexed as a male. Dave’s specimens met that criteria. The assignment of Dave’s spiders to the Cyrtaucheniidae family was more complicated. That determination hinged on the analysis of five morphological characters that, fortunately, were well depicted in Dave’s initial photograph. That analysis followed a dichotomous key prepared by Darrell Ubick, as set forth in Ubick et al. (2005), pp. 25-26: 1. The spider is a mygalomorph, as the jaws (chelicerae) are paraxial (they project outward from the face, with the fangs opening as rakes, in line with the longitudinal axis of the body, rather than laterally, like scissors); 2. The abdomen is not segmented, and the spinnerets are short (to the point that they don’t noticeably project posteriorly, beyond the abdomen); 3. The spider is clearly not a theraphosid (which we in North America commonly refer to as a tarantula); 4. The abdomen is not truncated and sclerotized, as in the Cyclocosmia; 5. The thoracic furrow is transverse, not strongly procurved as in the Ctenizidae; The foregoing leads, by default, to the Cyrtaucheniidae family. Species of Cyrtaucheniidae in the genus Eucteniza are fairly common in South, Central, North, and EastTexas. They differ from Dave’s specimens, however, in being dark brown or black, while his are a light, reddish brown. Only two species of Eucteniza are known to have been found here, and neither has the light brown coloration of Dave’s spiders. For those who may be interested, individual articles featuring trapdoor spiders in the genus Eucteniza found in Austin, Travis County, TX, Kempner, Bell County, TX; San Antonio, Bexar County, TX; and Cedar Creek, Bastrop County, TX, are posted elsewhere on BugsInTheNews.info. Click on each of the links to view those articles. Returning to Dave’s spiders, a chapter on the Cyrtaucheniidae, authored by Jason E. Bond, in Ubick et al. (2005), pp. 45-47, provides a key to the family. Bond’s key, for male specimens, begins by requiring the investigator to establish whether the palpal tibia is provided with a retrolateral distal flange. A separate paper published by Bond & Platnick (2007), points out that this is best viewed ventrally, but inasmuch as Dave’s photo only depicted the dorsal body of one of his specimens, it was not possible to determine if the character cited by Bond was present. As noted below, I boldly asked Dave for additional photos, which — after going to great pains — he kindly supplied. The specimens, he told me, were quite active, and unlike trained puppy dogs were not of a mind to roll over on command. I explained that he could place them under refrigeration until they became temporarily paralyzed by the temperature; then it would be possible to invert them to take photos of their ventral bodies. Dave did so (though it was necessary to place them in the freezer for a short period, as they’d continued to run about with abandon even after half an hour in the refrigerator), and was then able to obtain excellent images while the spiders were thus incapacitated. Afterward, both specimens revived without any evidence of injury. The narrative related to those photos continues below, following a brief digression on the history, geology and geography of the setting where Dave collected these spiders: Dave’s home is near the city of Cresson, Texas. The city, positioned at the intersection of U.S. Highway 377 and State Highway 171, is about 17 miles south of Fort Worth, and sits astride the Hood-Johnson county line. According to one popular account, Cresson was named for one John Cresson, captain of a wagon train that camped in the area in the 1850’s. The area has, since the earliest of times, been mostly a ranching community. According to Spearing (1991), p.241, Cresson is located on a ridge crest of hard, Duck Creek limestone. This formation spans the Phanerozoic, Mesozoic, and early Cretaceous periods, rests on Kiamitia clays, and is succeeded above by Fort Worth limestone, which to some geologists forms an undivided formation with Duck Creek. The combined formation is known for a distinct faunal character. The Duck Creek and Fort Worth beds are rather well burrowed, with marine megafossils of Pecten, oysters, echinoids, and ammonites. Specific fossils of Kingena sp., Ostrea sp., Plictula dentonensis, Avicula sp., Inoceramus comancheanus, I. munsoni, Pholadomya sp., Pachydiscus brazoensis, Schloenbachia belknapii, S. acutocarinata, and Hamites fremonti have been recovered. On my visit to Dave’s home on 28 February, I observed and photographed a few ammonite specimens in Dave’s back yard. He later confirmed that he’d found them scattered in the yard shortly after closing on the property and moving in. They are pictured in the image montage above. As might be expected, the soil here is generally alkaline, and supports hardy, drought-resistant prairie grasses that do not require deep, high-nutrient profiles. Drought tolerant deciduous hardwoods, particularly those in the Cannabaceae and Ulmaceae families, along with evergreens in the Cupressaceae family, generally thrive in such soils. Dave’s home is positioned on the slope of a prominence that the tract rises toward in front, and slopes gently away from in back, the latter toward a creek thickly lined with a riparian deciduous hardwood forest. That forest is populated mostly with hackberries (Cannabacea: Celtus spp.) and elms (Ulmaceae: Ulmus spp.), interspersed with a few junipers (Cupressaceae: Juniperus spp.) The land here is generally a rolling prairie clothed with short thick stands of native grasses, with small areas of bare soil. Behind his home is a stand of deciduous hardwoods, including hackberry and elm, depicted above. One one side lies an extensive meadow, in which can be seen a few junipers. The native short grasses here cover the ground so thoroughly that manual searches for minuscule trapdoors would be quite tedious. However, since these spiders often build their burrows near the crowns of trees, the hardwoods behind his home offer a good chance of finding a few. Unfortunately, on my 28 February trip there was little time available for a thorough search, and in the scant time allotted I found none. Termites (shown above in a knob of wood) were found infesting a source of cellulose partly buried in the soil of one bare spot behind Dave’s home, as well as ants and grass spiders, ground beetles, and earwigs, suggesting that Dave, who is a physician, is laudably disinclined from the use of broadband pesticides in his yard. Were that not the case, it is doubtful these spiders would have been observed near his pool. On this trip, what was observed here appeared as a rich, undisturbed, native fauna. As mentioned earlier, on 24 February, Dave sent photos of the ventral aspects of his spider’s pedipalps. On examining these photos, both palpal tibias were found to possess the retrolateral distal flange alluded to in Jason Bond’s key. This structure has the form of a notably sclerotized ledge on the retrolateral — that is, the posterior — surface of the palpal appendage, distally. Huber (1995), studied similar structures — retrolateral tibial apophyses — in a grouping of entelegyne spiders, and concluded they serve to orient the embolus in the epigynum during copulation. I speculate (perhaps incorrectly) that the structure has a similar function for Dave’s specimens. In any case, the presence of a retrolateral distal flange is unique to the genus Myrmekiaphila, at least within the Cyrtaucheniidae. In the closely related Ctenizidae family, a somewhat similar structure is found in the palps of trapdoor spiders in the genus Bothriocyrtum, but there the morphological character is more a knob than the well-defined ledge observed in the Myrmekiaphila. Inasmuch as Dave’s spiders could safely be assigned to the genus Myrmekiaphila, that finding — combined with the location where the specimens were collected — might well be considered sufficient cause to assign them to the species M. comstocki without further ado. It happens that M. comstocki is the only species in this genus known to have been found within Texas in the past. In any case, Dave’s new photos, while excellent in all respects, did not provide sufficient information to enable a definite identification, beyond the presumption just mentioned. Circumstantial evidence, however, is a poor basis for good judgment. Thus, being in possession of the 2007 paper by Bond & Platnick, it seemed a waste not to take this investigation further, analyzing Dave’s specimens according to all the relevant morphological characters depicted therein. Investigators of the caliber of Jason E. Bond and Norman I. Platnick labor diligently to prepare and publish good, detailed taxonomic reviews that enable others to participate fully in the advance of science. Those others, enabled now to follow along in their footsteps, are elevated thereby to see more distant horizons. The analyst in the field who makes the effort to rise up and peer attentively beyond gives honor to their work. By failing to take advantage of such resources, those same analysts do them, instead, a dishonor. That I could not do. I’ve not yet had the pleasure of meeting either of these gentlemen, but in January of 2010, over a bowl of gumbo at a Round Rock, Texas restaurant by that name, Pierre Paquin and a colleague, Kemble White, regaled me with stories about their favorite arachnologists. Herb Levi, the dean of modern North American Arachnologists, topped the list. As Alexander Agassiz professor of zoology and curator of arachnology at the Museum of Comparative Zoology, Harvard University, Dr. Levi has authored some 150 scientific papers on spiders and on biological conservation. Always ready to help a colleague, of whatever station, both in or out of academia, Dr. Levi’s advice and consultation is instantly made available. Around the world thousands of scientists owe this humble, gracious, hard working friend a debt of gratitude. Next came Norm Platnick, close behind, and for Pierre, the reasons were personal. He and a close friend, Nadine Dupérré, became enthralled with the field of arachnology together, and while he tackled the sytematics, she became one of the world’s foremost illustrators of zoological specimens. Her drawings fill the pages of Ubkck et al. (2005) on North American Spiders, as well as a recently published book on the spiders of Quebec; without her special touches, those and many other publications would be sorely lacking. Since 2008, she has worked at the American Museum of Natural History (AMNH). There she serves as Dr. Platnick’s scientific assistant, sorting, data basing, imaging, and crafting illustrations of, among other things, spiders in the Oonopidae family. Pierre explained how pleased he was that she had the opportunity to work for a luminary of Dr. Platnick’s stature. Platnick received his PhD at Harvard in 1973 after authoring a revision of the North American spiders in the Anyphaenidae family. He is presently the Peter J. Solomon Family curator of the invertebrate zoology department of the AMNH. In that capacity he’s deeply engrossed in a highly ambitious taxonomical revision of the oonopids, working with a group of 30 investigators from ten countries to assemble a Planetary Biodiversity Inventory (PBI) on this curious family of minuscule, haplogyne, araneomorph spiders. Species in the Oonopidae are tiny spiders, and usually have six eyes, but many have four, and a few but two. Some are suspected of being parthenogenetic, as — for their species — no males have been found. At that January 2010 dinner meeting, Paquin and Kemble didn’t mention Jason Bond. Our focus that night was on blind troglobites, rather than trapdoor spiders, which may explain why his name didn’t come up. I hope to meet Dr. Bond one of these days though, and ask him how many requests he’s received to strike a pose and say “The name is Bond… Jason Bond.” No doubt he’ll admit it happens too often, and has been a bit irksome. Still, he seems like the kind of fellow who might enjoy such trifles. Who else would name a species of trapdoor spider (Myrmekiaphila neilyoungi) after a folk singer? Or another (Apostichus stephencolberti) after a comedian and political satirist? Professionally, Bond is a professor in the Department of Biology at East Carolina University, Greenville, North Carolina, and a leading authority on mygalomorph spiders in the Ctenizidae and Cyrtaucheniidae families. The lab at ECU that bears his name focuses on speciation and adaptive radiation, and carries out alpha-taxonomic (finding, describing, and naming species of living or fossil organisms), phylogeographic (historical effects responsible for contemporary geographic distributions), evolutionary, and spatial distributional studies of spider and millipede populations and species. In any case, given the high respect these distinguished arachnologists are due, I made arrangements to visit Dave’s home on 28 February to collect his spiders for a more detailed analysis in my lab, so that they could be properly compared with the morphological characters described in their taxonomical review. Some may wonder why I didn’t just pickle Dave’s specimens up and ship them out to one of these scientists. Their labs are much better equipped than mine, and are populated with bright young minds eager to tackle such challenges. Without doubt, all of that is true. However, bright young minds, in sparkling, well-equipped laboratories, don’t sit around waiting for challenges to jump. Such labs —and those who man them — are busy as beehives, churning out research products from laundry lists miles long. Who am I to beg them to turn from their present labors to attend to this minuscule project? And besides, passing the buck means missing out on the fun. Judith Winston (1999), pp. 10-11, puts the whole issue into perspective with these words: “… professional systematists … have their own research programs to carry out, to which they are probably already overcommitted.” She goes on to say, on p. 11, “In summary, most biologists who find a new species, whether living or fossil, must describe it themselves.” Fortunately, her book, along with published articles of the ICZN, provides the foundation on which non-systematists may do such things on their own. Thus armed, I dug into the work. The findings produced from my analysis are described below: The palps of Dave’s specimens, as photographed under a dissecting microscope on 1-4 March 2011, resemble those of M. comstocki in a number of important respects. In particular the palpal tibia is rather robust in Dave’s specimens (rather exact measurements of this and other characters, using a Bausch & Lomb photogrammetric microscope equipped with a built-in reticle, are in process and should be published soon). This character is cited in Bond & Platnick (2007), p. 11, to distinguish M. comstocki from M. foliata. However, there appear to be significant differences as well, to the point that certain affinities with M. foliata, a species that has not been observed in Texas but is endemic to the southeastern states of Alabama, Georgia, Tennessee, Kentucky, and North Carolina, are striking. Those differences include the presence of a sclerotized edge of the retrolateral distal flange (RDF). Images in Bond & Platnick (2007), p. 4, of the RDF for M. foliata (ibid, fig 4) seem to depict a similar darkening of this morphological character, which is not present in images of M. comstocki (ibid, fig. 5). No mention of this distinction is made in the referenced paper, which may mean that the authors, both of whom are much more experienced with these spiders than I, considered it irrelevant. Another difference found in Dave’s specimens is the presence of a relatively large distal dilation on metatarsus I, bearing four stout ventral spines. Again, on p. 9, the presence of (usually) more than two ventral spines, on a relatively large dilation of metatarsus I, is cited to distinguish M. foliata from M. comstocki. In specimens of M. comstocki, the metatarsal swelling is much reduced, which appears — to my relatively inexperienced eye — quite unlike that of Dave’s specimen. Again, exact measurements of this character are in process. Incidentally, for readers who may wonder, the metatarsal swelling, and the narrowing of the proximal and middle portions of the metatarsus, form a critical portion of this spider’s mating claspers. They are not present in the female, but the male uses them to grasp and hold the female during the mating process. With all these similarities to M. foliata, I was prepared to witness a strong similarity with that species in the morphology of the embolus of Dave’s specimens, particularly the presence of a prominent distal tooth. That tooth, while apparent, does not appear in Dave’s specimens in the exact form described and illustrated for M. foliata (ibid, fig. 24, p. 7). The emboli of Dave’s specimens are observed to have two pairs of diminutive teeth distally, the most distal pair being on a prominence similar to that described for M. foliata, preceded proximally by, first, the other pair of diminutive teeth, thence by three or more larger teeth. From the aspect of the (ventral) image at left, the most distal set of paired teeth seem to be oriented longitudinally with respect to each other. However, as shown in the semi-ventral aspect, depicted in the final image in the photo montage above, these appear to be paired transversely, as in a scrape-awl or nail remover, that has been fitted with a v-shaped transverse blade. In another perspective (as yet unpublished) the teeth seem to be aligned longitudinally, in close proximity. My lab microscopes are not presently equipped to image this on a stable frame, a deficiency for which I am seeking a remedy. As soon as that is done, better images will be posted. Either way, though, the pattern of these teeth is not consistent with that described (ibid, p. 9) for M. foliata (ibid, p. 11), M. comstocki, or M. coreyi (the next most likely candidate within the list of presently described species). In the case of the former, the character is described as an “enlarged, retrolaterally directed, sub-distal tooth on the embolus.” Had this tooth been observed in the form we see here, it seems doubtful that the pairing would be omitted from the description. The teeth on the embolus of M. comstocki, by comparison, is described as “lacking a prominent distal tooth (ibid, fig. 25)”; an examination of the cited figure shows the embolus to have 9 or more subequal, rather diminutive teeth. As a result of these observations, it seems wise to consider the possibility that Dave’s specimens are neither M. comstocki nor M. foliata. They may, in fact, constitute a separate species not previously described. As a courtesy, I sent an inquiry, via e-mail, to Jason Bond, and Norman Platnick, seeking their views on this question. Dr. Platnick, due to the obligations of his study on the goblin spiders, sent his regrets and deferred to Dr. Bond. He, too, may understandably be unable to take a look at this material for some time, but I am particularly interested in his views, and hope he will be able to provide some insight. The morphological disparities noted above do not necessarily demand the recognition of a new species. However, they appear, at minimum, to suggest a revision may be needed of the recognized morphological distinctions between M. comstocki and M. foliata. In any case, should it be deemed appropriate to designate this as a new species, I am proposing the specific epithet Myrmekiaphila petersi, in honor of Dr. David Peters, M.D., without whose native curiosity, combined with a diligent collection and imaging effort, this project would not even exist. Wherever this road takes us, I intend — with the help of others — to compile the necessary documentation to see it through. We (you and I, together: remember, this is a viewer-participant website) are here embarking on an alpha-taxonomic expedition of discovery. Along the way we will dig deeply into Judith E. Winston’s valuable reference book, “Describing Species: Practical Taxonomic Procedure for Biologists.” Simultaneously, we’ll pore over the International Code of Zoological Nomenclature, published by the ICZN, and do as commended by that distinguished committee. The specimens already collected will be preserved in accordance with accepted practices, and —when and where appropriate and necessary — submitted in the accepted manner as voucher specimens for others to examine and study. Dr. Peters has kindly offered to keep on the lookout for additional specimens, and is prepared to collect and preserve them for this project. I am planning additional visits to his home as well, to scout for burrows in the landscape, and collect females of the species. This work will be underwritten by the recently established Megatherium Society, and will constitute its first serious scientific project. As such, every step along the way will be documented and published in the annals of the society, in a form intended to help all who are interested in finding, describing, analyzing, learning about, and naming organisms. The work of scientific discovery is not confined to full-time scientists in our colleges and universities, but must be carried out by all those who love nature, are possessed of a curious mind and a desire to advance the search for truth and the dissemination of knowledge. These objects not only form the foundation on which the Megatherium Society rests, they also give it wings and enable it to fly. The genus Myrmekiaphila was named in 1886 by George Francis Atkinson, an agricultural botanist whose work led him to study the ant fauna inhabiting cultivated farmland. He found specimens of these spiders closely associated with ant nests in the soil. The generic name he applied to them, having as its prefix the Greek μυρμεξ, myrme(x), “ant,” and the Greek suffix φιλος, philo(s), “loving,” means “(one that) loves ants.” This article is in process. Additional photos and narrative will be posted soon. REFERENCES:
jc —————————————– — BugsInTheNews is a VIEWER-PARTICIPANT WEBSITE.Questions? Corrections? Comments? BUG ME RIGHT NOW! Telephone Jerry directly at 512-331-1111, or e-mail jerry.cates@bugsinthenews.info. You may also register, log in, and leave a detailed comment in the space provided below. |